Fascia, a fibrous connective tissue, is responsible for allocating skeletal muscles and corresponding neurovasculature into function-based compartments.
Pressure is capable of accumulating within these compartments, initiating the onset of a condition commonly referred to as compartment syndrome. When the pressure within the compartment compromises the arterial supply, it prevents perfusion of oxygen into the surrounding tissues, leading to tissue necrosis, or tissue death. Once diagnosed, a fasciotomy must be performed immediately to prevent severe complications.
A recent study evaluated the incidence of fasciotomies performed during Operations Enduring Freedom and Iraqi Freedom and found that of 4,332 casualties to the extremities, 669 (15%) underwent a fasciotomy. According to the Joint Special Operations Medical Training Center (JSOMTC), the current training methods are insufficient for practicing the surgical technique, while other methods, such as cadaveric and live tissue training, are cost-prohibitive for the number of students that require annual training. The U.S. Army Research Laboratory Human Research and Engineering Directorate Advanced Training and Simulation Division (ARL HRED ATSD) identified the requirement to develop a next-generation lower extremity fasciotomy Part-Task Trainer (PTT) in response to JSOMTC’s need for a more realistic, durable, and cost-effective training approach. The paper will describe the research conducted to satisfy these requirements, including identifying, developing, and validating the essential anatomy and physiology required to provide a realistic and effective next-generation surgical PTT. Additionally, the paper will explore how innovations in novel synthetic materials provided realism approaching traditional methods while greatly minimizing cost and maximizing training opportunities.
INTRODUCTION
A fibrous connective tissue, known as fascia, surrounds human skeletal muscle, encasing it and the corresponding neurovasculature while separating these tissues into compartments. Fascia is tough and inelastic, which introduces pressure in the muscle compartments in response to a traumatic event or collection of fluid. As the pressures increase in the muscle compartments, the arterial supply and coinciding perfusion rates are compromised, leading to tissue necrosis, or tissue death. The etiology of acute compartment syndrome often stems from traumatic injuries commonly seen during combat, such as fractures or blast injuries. Presentation of compartment syndrome includes pain, palpable tightness, tenderness, paresthesia, and paralysis, and once diagnosed, a fasciotomy must be performed immediately to prevent further complication (Compartment Syndrome (CS) and the Role of Fasciotomy in Extremity War Wounds, 2012). Todd Ulmer (2002) discovered that if one of these clinical symptoms is present, there is a 25% chance of the patient having compartment syndrome; this percentage increases to 93% if three of the aforementioned clinical findings are present. Evaluation of the clinical signs and symptoms is the preferred method of diagnosing acute compartment syndrome; however, testing the pressure within each myofascial compartment is becoming increasingly common as an additional diagnostic method. If treatment is delayed, complications such as muscle excision, amputation, and mortality can arise, with some of these complications occurring less than six hours after onset of increased compartmental pressure (Compartment Syndrome, 2017). This time constraint sometimes expires before a patient can be evacuated, requiring that Special Operations Medics be trained to perform the procedure if a surgeon is unavailable.
Kragh et al. conducted a study that evaluated the incidence of fasciotomies performed during Operation Enduring Freedom and Operation Iraqi Freedom. The study found that of 4,332 casualties to the extremities, 669 (15%) underwent a fasciotomy (Kragh et al., 2011). Another study found that fasciotomy rates have been significantly increasing over time. They determined that in 2001, no casualty reported to a military trauma registry received a fasciotomy, whereas in 2010 approximately 26% of 17,166 reported casualties received a fasciotomy (Kragh et al., 2016). Previous studies have demonstrated that ample training and experience are essential to performing a successful fasciotomy. Current training methods include video instruction, classroom training, and educational programs to aid in diagnosing the condition (Kragh et al., 2013). According to the Joint Special Operations Medical Training Center (JSOMTC), the current training methods of instruction for the fasciotomy procedure, such as cadaveric and live tissue training, are inadequate and cost-prohibitive.
TECHNICAL OBJECTIVES
JSOMTC asked the U.S. Army Research Laboratory Human Research and Engineering Directorate Advanced Training and Simulation Division (ARL HRED ATSD) to evaluate the feasibility of developing a realistic, durable, and cost-effective lower extremity fasciotomy Part-Task Trainer (PTT). Other studies have validated this requirement in stating that more research is needed to improve clinical performance for fasciotomies and continued research is necessary to investigate valid alternatives for live tissue training (Kragh et al., 2013 & Sakezles et al., 2008).
The need for a more realistic, durable, and cost-effective training approach highlighted the urgency for a nextgeneration fasciotomy PTT to address:
- Relevance: the occurrence of fasciotomies performed on casualties to the extremities during Operation Enduring Freedom and Iraqi Freedom was 15%.
- Insufficient training methods: according to the Joint Special Operations Medical Training Center (JSOMTC), the current training methods (video instruction and educational programs) are insufficient for practicing surgical technique.
- Cost-efficacy: Other training methods, such as cadaveric and live tissue training, are cost-prohibitive for the number of students that require annual training.
In response to the need, the team outlined the following research aims and objectives:
- Identify, develop, and validate the essential anatomy and physiology required to provide a realistic and effective next-generation surgical PTT for the treatment of lower extremity compartment syndrome.
- Research and develop new synthetic materials and methodologies that provide realism similar to cadaveric or live tissue training.
- Minimize cost and maximizing training availability while ensuring a durable and easily refurbished product.
- Validate the PTT design and training relevance through consultation with Subject Matter Experts (SMEs) and usability studies.
REQUIREMENTS AND CRITICAL TASK ANALYSIS
The development team met with SMEs at JSOMTC and the Medical Simulation Training Center (MSTC) at Fort Bragg. The team elicited requirements from instructors at both training centers to better understand the Program of Instruction (POI), logistical constraints, and opportunities for improvement. The POI was reviewed and decomposed to outline the critical tasks and skills required to perform the procedure in order for the team to identify training gaps and opportunities for improving the current training model. Current training models include cadaveric and live tissue training as well as a fasciotomy training device that requires refurbishment by the manufacturer after each use. Due to the cost and time required to refurbish the existing fasciotomy trainer, it is no longer utilized as part of the POI.
Including essential and anatomically accurate landmarks in the PTT is critical to performing a successful fasciotomy. Identification and replication of these key landmarks orient the medical provider with the anatomy prior to making the initial incisions and ensuring that only the proper structures are cut during the procedure. Figure 1 demonstrates some of the key landmarks and structures that require identification for the fasciotomy procedure. If incorrectly identified, the provider may unintentionally cut important nerves or vessels that are identified as critical landmarks in the POI. To begin the procedure, the shaft of the fibula is the first anatomical landmark identified. The provider must palpate the proximal and distal ends of the fibula to locate the shaft of the long bone, from which the lateral incision should be made two centimeters anterior. After making the lateral incision, the anterior intermuscular septum must be identified; it separates the anterior and lateral compartments. The tibial crest, or palpable portion of the tibia, must then be identified so that a small horizontal incision is made midway between it and the anterior intermuscular septum. The superficial peroneal nerve must then be identified as it sits near the anterior intermuscular septum and is at high risk of being injured during this part of the procedure. Severing the superficial peroneal nerve could result in foot drop, which is a loss of the ability to pull the toes and foot upwards, and a loss of sensation to the anterior and lateral parts of the leg. A bony prominence on the lateral part of the ankle, referred to as the lateral malleolus, is identified next. The scissors are pointed toward the lateral malleolus as the fascia of the lateral compartment is cut to keep the instrument posterior to the nerve to avoid damage. For the medial incision of the skin, the posterior medial edge of the tibia must be palpated and the incision made two centimeters posterior to this landmark to avoid the great saphenous vein. During the anterior fasciotomy, the scissors are directed towards the patella, or kneecap, to avoid damaging the proximal neurovasculature to prevent the morbidities associated with nerve damage. SMEs at JSOMTC requested that the gastrocsoleal complex (composed of the gastrocnemius and soleus muscles) and its attachment to the posterior border of the tibia be included because it must be released to access the deep posterior compartment of the leg.
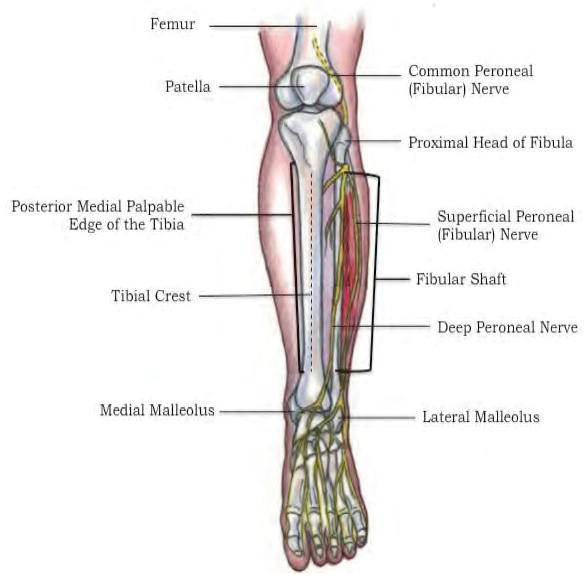
Figure 1: Landmarks and Structures for the Fasciotomy Procedure – Source: Author
Aside from the skin, muscles, and fascia, it is also important to include adipose tissue and neurovasculature of the lower leg. When surgeons cut through the dermal layers and adipose tissue, they must successfully identify the neurovasculature to avoid causing permanent damage to the patient. This can be one of the most challenging aspects of performing a fasciotomy. Proper tactile feedback and anatomical accuracy will aid in creating essential muscle memory for those who train for fasciotomies. If there is damage to a nerve or vessel, the patient can bleed excessively or have a loss of function in certain muscles possibly coupled with a loss of sensation in the skin. These potential morbidities can lead to a decreased quality of life for the patient, or even amputation of the affected limb. Figure 2 demonstrates the incisions for a four-compartment fasciotomy of the lower extremity.
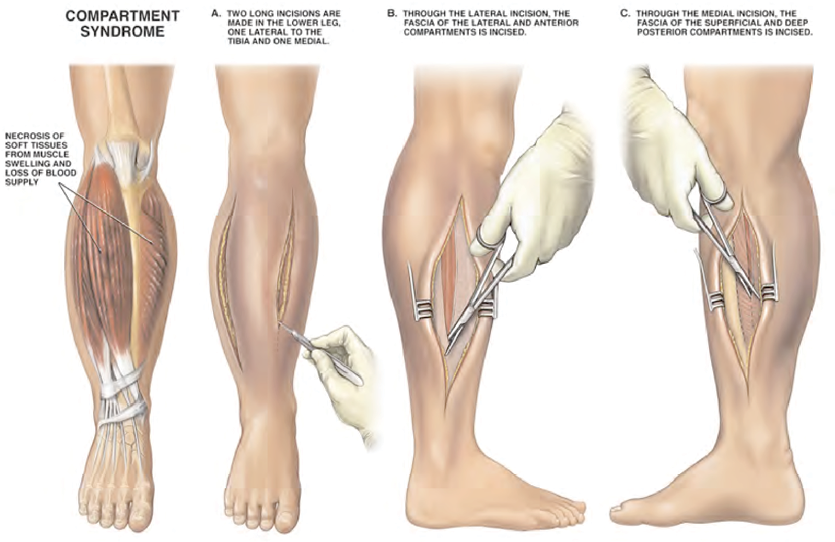
Figure 2: Demonstration of Fasciotomy Incisions – Source: Nucleus Medical Art, Inc. (2001,
February 1). Four Compartment Fasciotomy Procedure [digital image]. Retrieved from: http://www.
alamy.com/stock-photo-four-compartment-fasciotomy-procedure-7711954.html
A market survey of the current fasciotomy trainers was conducted to understand the state of the art and limitations of modern training systems. The market survey compared the commercially available fasciotomy trainers to the requirements elicited from SMEs and the POI. Meetings with SMEs were held to confirm the initial critical task analysis conducted by the team after evaluating the POI. These meetings were informal but included detailed discussions with training aides as well as demonstration of the fasciotomy procedure. Factors such as anatomical accuracy, refurbishment, and reusability were considered during the market survey, which concluded that none of the existing training devices evaluated were found to realistically simulate the fasciotomy procedure of the leg while meeting the requirements associated with cost and refurbishment time. Multiple training devices were software based and did not provide hands-on training, while others were not field-serviceable, requiring that they be sent to the manufacturer for refurbishment after a single use.
MATERIALS RESEARCH AND DEVELOPMENT
Realistic appearance and accurate tactile feedback are necessary to effectively train emergency medical personnel on the fasciotomy procedure. It is also crucial that the PTT is durable, has the capability of being used and refurbished in the field, and is easily stored when compared to live tissue or cadaver training. These considerations lead the team to explore new and existing materials and methodologies that offer the desired results. Materials commonly used in the industry, as well as readily available everyday materials were tested to evaluate options for all required simulated tissue and anatomical structures of the PTT.
Skin, adipose, and muscle tissues were the first to undergo testing. Silicone was the first material evaluated because it is often used in the industry and it offers characteristics that align with the requirements of the training system. Several samples of silicone rubber were evaluated based on durometer, tear strength, and the time and cost to manufacture the samples. Silicone foams were assessed based on their viscosity and volume expansion. Silicone is flexible, strong, compatible with other materials, easy to shape, compatible with pigments, and can exhibit fine details. It also has self-healing qualities and the ability to be repaired, which were favorable characteristics for the fasciotomy PTT. The team evaluated gelatin as another option for the skin, adipose, and muscle components of the PTT. Gelatin is inexpensive, compatible with other materials, demonstrates a high level of detail, and can be resealed with heat. Unfortunately, gelatin can be distorted at extreme temperatures or when used with fluids, which were both considerations for the fasciotomy PTT. Although the PTT is designed for a classroom setting, this characteristic could pose a conflict when considering storage and long-term use.
Fascia is a thick, dense connective tissue that differs from skin, adipose, and muscles tissues. A variety of materials, including commercially available simulated fascia, were evaluated for the PTT. Fascia separates the four muscle compartments of the lower extremity as depicted in Figure 3. It is sensitive to hydrostatic pressures and demonstrates minimal, if not absent, elasticity. Fascia is slightly translucent, pearl-white in color, and must be strong enough to contain the pressure that accumulates during an acute compartment syndrome, taut enough to fit around the defined muscle compartments, and flexible enough to be easily replaced after a training session. Materials such as vinyl, silicone, mesh fabrics impregnated with silicone, silk impregnated with silicone, latex, and shrink-wrap were evaluated based on the previously mentioned properties of fascia. The requirements for fascial tissue proved challenging, as most samples of simulated fascia demonstrated too much elasticity or were too stiff to conform around the muscle compartments. SMEs provided feedback regarding appearance and tactile response after completing the procedure on an initial prototype (which requires cutting the fascia); their feedback was used to adjust the formula and combination of materials used to simulate fascia more accurately. This iterative process resulted in highly realistic fascia, both in terms of appearance and response to cutting and stretching.
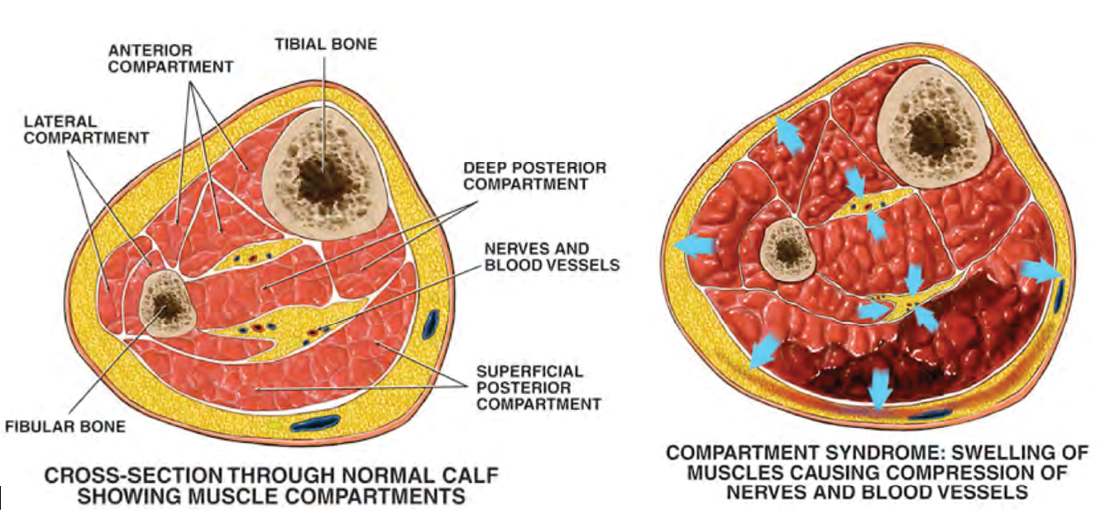
Figure 3: Lower Extremity Compartment Syndrome and Muscle Compartments – Source: Nucleus Medical Art, Inc. (2001, January 3). Compartment Syndrome
with Fasciotomy Procedure [digital image]. Retrieved from: http://www.alamy.com/stock-photo-compartment-syndrome-with-fasciotomy-procedure-7712213.html
Nerves, veins, and tendons are often encased by adipose tissue for protection or are found just beneath the fascia, making their tactile feedback and authentic appearance necessary for landmark identification. As a result, the team evaluated materials that could be used to simulate these vital structures during the fasciotomy procedure. It was important that the simulated structures were similar in tensile strength to the human tissue counterparts to provide a realistic sense of injury during training scenarios. Different varieties of silicone, some embedded with additional materials, were evaluated for the role of veins, nerves, and tendons in the PTT. The samples were evaluated based on appearance, tactile feedback, and tensile strength by physicians and SMEs who provided subjective feedback during informal interviews and after physically examining the simulated tissues.
The University of Central Florida (UCF) Advanced Materials Processing and Analysis Center (AMPAC) conducted basic research to simulate the bleeding caused by incision through the superficial capillaries as depicted in Figure 4b. AMPAC developed a proof-of-concept synthetic skin using hydrogels and lipid nanotube technology. Figure 4a shows an example of the simulated blood embedded in the hydrogel under the skin layer. When the skin layer and matrix of the hydrogel is severed, the entrapped simulated blood is released, giving the appearance of bleeding or oozing. The hydrogel concept provides realistic behavior but requires complicated formulas and processes to produce the desired results. The team performed a cost-benefit analysis to determine if this is a desired capability for the PTT and concluded that the manufacturing and lifecycle/sustainment costs exceeded the target costs recommended by the JSOMTC SMEs for the PTT. The materials and methodologies required far exceeded the cost of the current training devices since they required specialized skill, equipment, and materials that are not easily sourced or available on the open market.
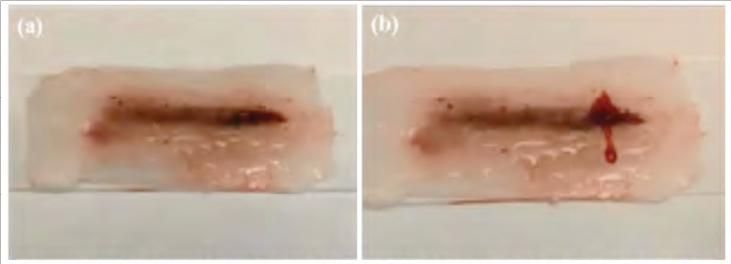
Figure 4: Hydrogel-Embedded Skin (a) Before; and (b) After Cutting – Source: Author
PROTOTYPE DEVELOPMENT
After initial meetings with SMEs at JSOMTC, the team developed a concept for the PTT that met their training requirements, constraints, and training gaps. The SMEs primarily rely on a live tissue or cadaver model, which limits the level of realism (in the case of the live tissue model) and access to training (in both cases). The prototype concept was decided to be a standalone PTT that didn’t require specialized storage or tooling to reset it between uses. Initial concepts were sketched, as depicted in Figure 5, to illustrate design and concept of multiple layers of anatomical structures that could be used to teach the procedure from start to finish. The initial prototype was meant to support the evaluation of different simulated tissue samples, but instead resulted in a proof of concept PTT that also provided SMEs with the opportunity to provide feedback regarding how the PTT addressed the requirements as well as simulated the landmarks, structures, and tissue.
Figure 5: Initial Sketches of Concept Multi-Layered Design – Source: Author
The initial design of the prototype included a foot that was fixed in place at the inferior end, and a partial knee at the superior end. Simplicity was a key design consideration for the PTT (Figure 6) because it could impact cost and refurbishment. Some training systems evaluated during the market analysis require they be sent back to the manufacturer to be refurbished. The development team designed the PTT such that it can be refurbished rapidly with minimal effort to maximize available training time and limit additional instructor workload. Commercially available components, such as the simulated tibia and fibula bones for the core of the trainer, were sought out when appropriate. The commercially available simulated bone adds to the realism of the PTT due to the extraordinary level of detail provided by the manufacturer. Due to a shortened development cycle, the muscles on the initial prototype were grouped together for each of the four muscular compartments and surrounded by a single outer fascia to facilitate SME feedback. This design simplified refurbishment and was approved by SMEs for utilization in the continued development of the prototype. The highest level of detail was devoted to the anatomical structures with which the trainee will interact with when performing the fasciotomy including procedural landmarks, fascia, and neurovasculature. The structures are inclusive of those previously mentioned as important landmarks for the procedure. Fluids were not integrated into the initial prototype and after receiving feedback from SMEs, it was indicated that fluids were not necessary and could add additional sustainment and lifecycle cost consideration with little added training benefit.
The ability to use the PTT to simulate compartment syndrome was another key requirement. Controlling the amount of pressure within each muscular compartment provides trainees with the ability to learn how to identify compartment syndrome by palpating a rigid compartment under extreme tension. Although the initial prototype required that the muscle compartments be inflated manually, it did allow for each of the compartments to be inflated individually permitting instructors to teach trainees to examine, assess, and treat compartment syndrome in patients. Initial designs to simulate increased pressure within the muscular compartments involve insertion of an air bladder within the simulated muscle, which allowed for incremental increases in pressure. The initial air bladder design proved challenging and required a reinforced design to be developed. Another method considered for simulating compartment syndrome was to mold muscles for a patient under normal conditions and another set of muscles for a patient with compartment syndrome. This method, however, would increase the cost for the user and potentially pose for a more difficult refurbishment process. The team continues to investigate other methods of simulating compartment syndrome as well as improvements in air bladder design for future iterations of the PTT.
Although a significant amount of time and effort was placed on identifying the ideal simulated fluids for the PTT, the SMEs at JSOMTC determined that it was not necessary to simulate fluids, especially if that capability added cost and further complicated maintenance, refurbishment, and sustainment of the PTT. After performing a cost-benefit analysis, the added training value did not justify the additional cost. Based on the results of iterative testing and SME feedback, several simulated tissue samples developed for the fasciotomy prototype were eliminated as nonfunctional for the continuing development of the PTT. After evaluating several forms of gelatin in variable conditions, the development team determined that silicone is the preferred solution for the PTT. Silicone met several requirements for the PTT including cost-efficiency and durability. The gelatin, although initially promising due to its easy repair, proved problematic due to the changes when placed in liquid environments and extreme temperatures. The samples tested for nerves need further investigation, as the current samples were eliminated for use in the proof of concept PTT demonstrated in Figure 6. The initial samples evaluated were too durable and somewhat difficult to cut. Utilizing them in the trainer could result in negative training since they would be more difficult to cut than a real human nerve.
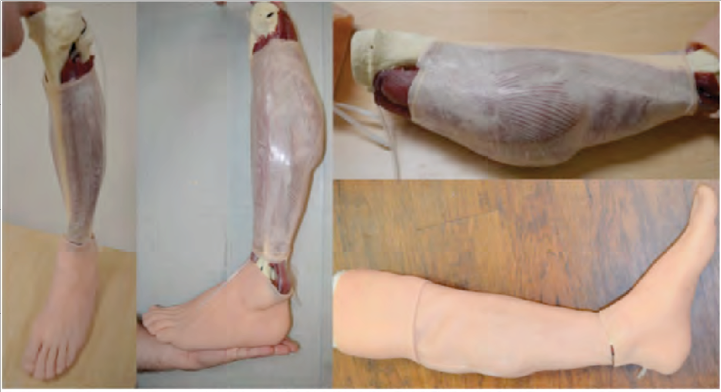
Figure 6: Proof of Concept PTT – Source: Author
CHALLENGES
The destructive nature of the procedure limits the number of potential options for refurbishment. Currently, the components of the trainer that undergo significant damage (e.g. skin and fascia) are completely replaced. Other layers, such as underlying muscle, nerves, veins, and tendons, will have the capability to be replaced if damaged. If the procedure is performed correctly, they should last indefinitely. Additional research and testing is being conducted on the viability of skin and fascia repairs to lower lifecycle costs of the PTT.
The air bladders that were integrated into the simulated muscle compartments proved to be unreliable because the air leaked from where the tubing attached to the silicone muscle. When the simulated muscle compartments were pressurized over a prolonged period, the bladder system would slowly deflate. An improved method and design will have to be evaluated that offers increased reliability for maintaining the simulated muscle compartments while pressurized.
SUBJECT MATTER EXPERT FEEDBACK AND EVALUATION
During a visit to JSOMTC at Ft. Bragg, the team received feedback from SMEs after performing a fasciotomy on the initial prototype of the lower extremity fasciotomy PTT (Figure 7). The development team asked the SMEs questions about the prototype’s design and conducted a focus group discussion about the components of the PTT. Vital feedback on the thickness of the skin, adipose, and fascial layers, as well as the bones, were provided to the development team. Several key items were identified and prioritized for addressing in the next iterations of the prototype. The skin and fascial layers were identified as being approximately 5% too thick. The skin on the proximal part of the trainer was identified as needing tightening. SMEs also recommended making the nerves less mobile to give them added accuracy and specified the importance of the attachment of the gastrocsoleus complex to the posterior border of the tibia, as previously mentioned. The remainder of comments made by SMEs were about a path forward into the following phases and additional capabilities. A second visit to JSOMTC focused on newly developed muscle compartments and fascia samples, which were reviewed and approved by the SMEs. It was stated that the focus of Phase II is on replacement at low cost and rapid refurbishment, while Phase III will focus on interoperability with existing Human Patient Simulators (HPSs) or manikins. A possible capability of the trainer would be to look at utilizing fluid filled bladders instead of air to potentially include ultrasound concepts for detecting a Deep Vein Thrombosis (DVT). Another easily executed capability would be the inclusion of multiple skin tones to represent different ethnicities. SME feedback and evaluations will be considered for continual development of the prototype during future phases.
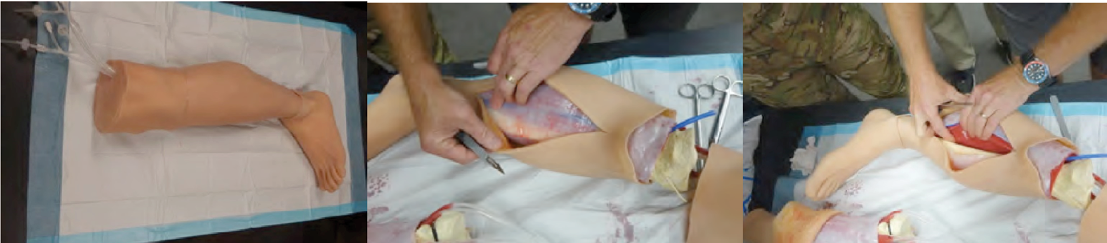
Figure 7: SME Testing and Feedback – Source: Author
SME input proved to be an asset in focusing the team’s development efforts. Although SMEs provided a wide variety of inputs, they were condensed through collaboration between the SMEs and the development team to enhance the focus on the key requirements for the PTT. The list below summarizes the primary and guiding inputs from the SMEs:
Reset or Refurbishment Time:
The ability to reset the PTT for conducting the next procedure in a short period of time is a primary concern of the SMEs. Initially a reset time of 5 minutes was requested, but after further discussions and use case exploration, being able to reset the leg in the field so that training could occur twice in the same day was acceptable.
Inclusion of Appropriate Landmarks:
Including soft tissues and underlying bone structures distal to the tibial plateau is required to provide landmarks for palpating and locating incision locations. At least 3” of anatomy (skin/bone) above the knee is required as a visual indicator for orientation purposes.
Muscle Compartments:
Development of muscles should be focused at the compartment level and not necessarily at the individual muscle level.
Skin and Adipose:
Development of the skin and adipose should be combined as they are cut and moved together, not separately. Thickness of the skin is also key and should be reduced if possible.
Fascia:
Ensuring that the fascia does not expand or has high resistance to stretching or expansion is a key characteristic of the material. A translucent material that allows the trainee to visualize underlying muscle allows for the proper appearance for the PTT.
The feedback gathered during sample and procedure testing was as essential as the guidance provided by the SMEs. By providing an initial prototype and iterative samples, the SMEs enabled the team to narrow material options and focus research and development on a decreasing subset until appropriate soft tissue representations were developed.
CONCLUSIONS
The team set out to fulfil the primary requirements of creating a PTT that is anatomically correct, accurately represents the conditions present in a lower extremity compartment syndrome, facilitates the execution of the fasciotomy procedure, and is easily and cost-effectively refurbished in the field. Systems engineering processes were applied which resulted in a singular, verifiable set of requirements that guided the creation of the PTT throughout the development lifecycle.
The team utilized iterative research and development techniques in the production of several different materials that make up the skeletal system and soft tissues of the PTT. Skin and adipose are vastly different from the fascia surrounding the muscle compartments, which is exceedingly different from the muscle tissue itself. The team constructed an outer skin and adipose layer that is realistic and reacts appropriately under conditions representative of compartment syndrome. The skin and adipose layer are silicone based and take advantage of years of research and development of simulated tissues undertaken by the team. The fascia is comprised of a unique material that resists stretching or expansion, is translucent, and provides for movement between surfaces. Development of the fascia consumed a significant amount of research and iterative development hours. The resulting fascia has a high degree of realism in visual appearance and tactile feedback, and received high praise from the SME evaluators. The underlying muscle tissues are also silicone based and were chosen due to the ability to add realistic details, as well as the expansion properties needed to accurately represent compartment syndrome. Several iterations of research and development of the muscle structures were required to perfect the internal bladders and overall function of the muscles.
Throughout the development cycle the team gathered feedback from Subject Matter Experts (SME) that proved invaluable in guiding and course-correcting the development of the PTT. Initial skin and adipose layers were modified to better represent the tissues and expected reactions when performing a fasciotomy. The fascia development was guided by SME input throughout the development cycle and resulted in a realistic fascia that is also cost effective. Finally, SME inputs helped identify the key requirements allowing the team to focus on the most critical aspects of the PTT development.
An anatomically accurate and realistic PTT has been developed through application of sound systems engineering processes, materials research, and incorporation of SME feedback throughout the development process. The final design is based on permanent and consumable components that represent the look, feel, and reactions of a lower leg presenting with compartment syndrome. The final design incorporates consumable skins and fascia developed for each muscle compartment that are replaced after each use. The rigid structures of the leg, its supporting components, and the soft muscle tissues are reused after each fasciotomy procedure is performed. The design centers on providing the appropriate landmarks for identifying where the incisions and cuts are to be made, and then the accurate look, feel, and reactions of the tissues and underlying structures as the procedure is performed.
PATH FORWARD
Additional research is needed to develop the materials for the PTT to increase the level of realism, anatomical accuracy, and physiological responses while considering cost, durability, and field refurbishment. Some of the tissue types, such as nerves, that were evaluated did not have any samples that successfully represented real human tissues with regards to the prototype. The materials used for these tissues will need further investigation and feedback to find a viable option to be researched and ultimately utilized in the PTT.
The development team will research the use of sensors embedded in vital neurovasculature structures to provide the capability to monitor and assess trainee performance throughout the duration of the training scenarios. The sensors would also support stimulation of physiological responses such as fluid release and changes in pulse rate, if practical. Self-healing capabilities of silicone will be further tested and evaluated to determine their applicability to a fasciotomy PTT, as current methods do not yet satisfy all requirements in this area. Although the tissues have been successfully repaired, the repair process is either extensive, or the marks from the initial use are still visible after repair. Since finding a balance between realism and low-cost replaceable components that are destroyed during the procedure is a significant challenge to the development of the prototype, self-healing or repairable silicone will continue to be investigated.
The development team will build upon the initial prototype and continue to develop and demonstrate a prototype PTT while engaging SMEs and the team’s extensive background in anatomical models for medical education. System requirements will be regularly updated and the PTT will be designed to reflect the updated requirements. The prototype will improve proof-of concept tissue layers and the initial prototype design and functionality.
ACKNOWLEDGEMENTS
The authors would like to acknowledge and thank the instructors at the JSOMTC for their continued support and feedback during the research and development effort of the initial prototype PTT.
REFERENCES
- Compartment Syndrome. (n.d.). Retrieved March 28, 2017, from http://www.mdguidelines.com/compartmentsyndrome
- Joint Theater Trauma System Clinical Practice Guidelines. (2012). Compartment Syndrome (CS) and the Role of Fasciotomy in Extremity War Wounds, 1-11.
- Kragh, J. F., Antonio, J. S., Simmons, J. W., Mace, J. E., Stinner, D. J., White, C. E., Blackbourne, L. H. (2013). Compartment syndrome performance improvement project is associated with increased combat casualty survival. Journal of Trauma and Acute Care Surgery, 74(1), 259-263. doi:10.1097/ta.0b013e31826fc71c
- Kragh, J. F., Dubick, M. A., Aden, J. K., Mckeague, A. L., Rasmussen, T. E., Baer, D. G., & Blackbourne, L. H. (2016). U.S. Military Experience From 2001 to 2010 With Extremity Fasciotomy in War Surgery. Military Medicine, 181(5), 463-468. doi:10.7205/milmed-d-15-00058
- Kragh, J. F., Wade, C. E., Baer, D. G., Jones, J. A., Walters, T. J., Hsu, J. R., . . . Holcomb, J. B. (2011). Fasciotomy Rates in Operations Enduring Freedom and Iraqi Freedom: Association with Injury Severity and Tourniquet Use. Journal of Orthopaedic Trauma, 25(3), 134-139. doi:10.1097/bot.0b013e3181e52333
- Sakezles, C., Moloff, A., Anton, J. J., & E. B. (2008). Advancing the Research and Development of Live Tissue Replacement Using Modeling and Simulation Technologies for Military Trauma Training (pp. 1-16, Working paper).
- Ulmer, T. (2002). The Clinical Diagnosis of Compartment Syndrome of the Lower Leg: Are Clinical Findings Predictive of the Disorder? Journal of Orthopaedic Trauma, 16(8), 572-577. doi:10.1097/00005131200209000-00006